Products
Scrap Copper Refining System Copper Cathode Production Line
- Copper is a good conductor of electricity, and is used extensively to make electrical wiring and components. The purification of copper is a form of recycling. Copper is purified
Copper is a good conductor of electricity, and is used extensively to make electrical wiring and components. The purification of copper is a form of recycling. Copper is purified further using electrolysis and we call it Copper Electrorefining (electrolytic refining).

In industry this is carried out on a massive scale. The electrolytic copper production is processed in the huge copper electrolytic tank fulfilled with copper electrolytic solution. Even the best chemical method cannot remove all the impurities from the copper, but with electrolytic refining it is possible to produce 99.99% pure copper (whatever method is used to manufacture copper from its ore, its final purification is by electrolysis).
Customer work site of copper electrolysis project: Figure 1 shows the cathode plate and figure 2 shows the cathode copper after electrolysis. It is extracted from copper mine in smelting furnace, contains 98.5% copper. It is the raw material of electrolytic copper production. While the final electrolytic copper product contains 99.99% copper in it.
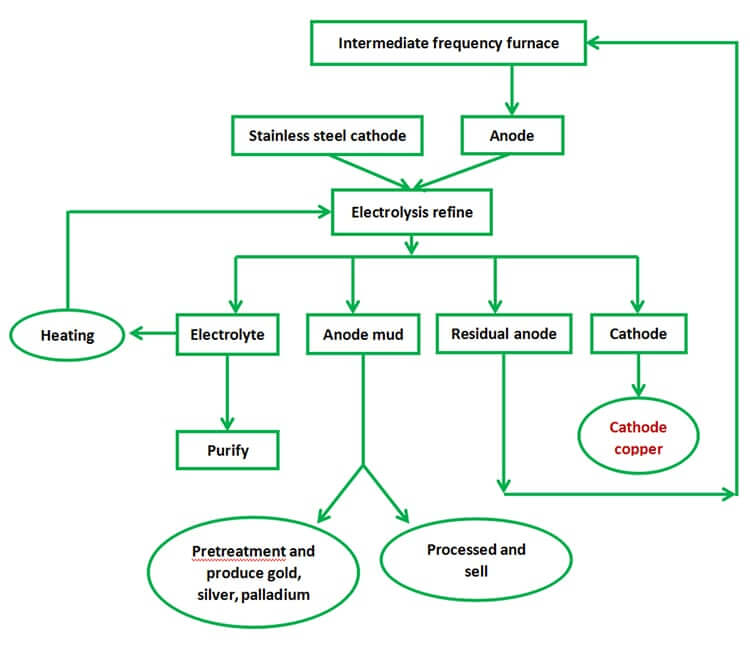
Working Flow
Copper electrolysis ,First, makes the crude copper which contains 98%-99.9% copper pouring into anode plate ,and use the pure copper sheet as cathode,then put them into the electrolytic cell
(which has the copper sulfate and sulfate solution);
Copper Electrolysis Refining Plant
Under the effect of direct current,the anode copper and poorer negative metal will be dissolved into the electrolyte,but the Gold,silver, palladium and other precious metals can't be dissolved, and they will be made into anode mud;

The copper which in the solution will be separated out preferentially, poorer metal that can't be separated out will stay in the solution ,they will be removed when purifying the electrolyte, that cathode will separate out more purer copper(99.99%), it will be called cathode copper or electrolysis copper.
-----Copper Electrolysis Refining Tanks
Anode plates are hung by their "handles" in electrolytic copper refining tank. Pure copper cathode sheets suspended on solid copper bars are inserted into the same tank, one sheet between each anode. When an electric current is passed from the anodes through the electrolyte to the cathodes, copper from the anodes moves into the solution and is plated onto the starter sheet. Impurities from the anodes settle to the bottom of the tank.

-----Copper Anodes(Plates) Casting Machine
The copper anode will be casting into anode plates in molds semi-automatically. After pretreatment removing of tin, lead, iron,aluminum starts charging of the copper material into the furnace which is followed by smelting process. When the impurities are removed follows the slag removal and phase of reduction with natural gas. Reduction is aimed to remove the free oxygen. After the reduction the process is ended by casting, where the final product is casted into the form of copper anodes.

-----Pure Copper Cathode Sheets
The refining anodes taken out from the refining furnace is changed into electrolytic copper with purity of 99.99% through an electrolysis process: During electrolysis, copper (II) ions leave the impure copper anode and since they are positive, migrating to the negative cathode. From time to time, the pure copper is scraped off the cathode. Impurities from the copper anode, such as gold, silver, platinum and tin, collect at the bottom of the electrolyte solution, deposited as anode slime.

-----More Details On The Impurities Here
Ag, Au, tin and Pt are more noble than copper, insoluble in this electrolyte and so do not deposit on the cathode. They will be found as metals in the anode slime;
Soluble impurities of iron and nickel dissolve in the electrolyte, which has to be continually purified to prevent excessive deposition onto the cathodes, which would reduce the purity of the copper;
Sn, Bi and Sb dissolve anodically but will precipitate in the electrolyte as oxide or hydroxide compounds which will be found in the anode slime.
Technical Parameters
| Model | RSKDJ-500 | RSKDJ-1000 | RSKDJ-2000 | RSKDJ-5000 |
| Mixed Metal Powder | 500 Kg/D | 1000 Kg/D | 2000 Kg/D | 5000 Kg/D |
| Energy Consumption | 280 | 570 | 1130 | 2850 |
| Electrolytic Cycle | 15 Days/48H | 15 Days/48H | 15 Days/48H | 15 Days/48H |
| Copper Capacity | 370 | 750 | 1500 | 3750 |
| Copper Purity | 99.99% | 99.99% | 99.99% | 99.99% |
NOTE:
Working voltage:380±10V(415V ,440V also can be customized);
(If you want more detailed parameters and quotations, please contact us)
LATEST NEWS
CONTACT US
Tel: 0086-13674945231(whatsapp)
Email: sunymachine@gmail.com
Add: Henan Communication Industrial Park) 5th St., Economic-Technological Development Zone, Zhengzhou
